Melasma is a common disorder of hyperpigmentation affecting millions of patients worldwide.1 It disproportionately affects women and patients with darker skin types and is associated with significant psychosocial morbidity.1,2 Despite the prevalence and impact of melasma, however, understanding of its epidemiologic presentation, risk factors, and treatment patterns remains limited. Thus, further clinical studies of validated cohorts are needed to better describe the natural course of the disease. To address this, we assessed the validity of using International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes for melasma to generate cohorts for retrospective study.
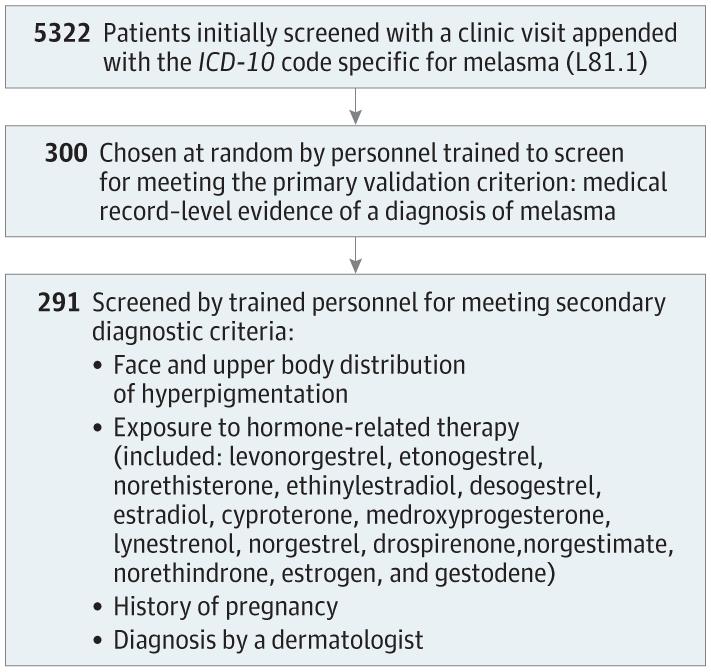
For this multi-institutional, cross-sectional study, we queried the Mass General Brigham Research Patient Data Registry for adult patients evaluated from October 2015 to January 2021 at Massachusetts General Hospital, Brigham and Women’s Hospital, Newton-Wellesley Hospital, Brigham and Women’s Faulkner Hospital, and associated satellite hospitals with a clinic visit appended with an ICD-10 code (L81.1) specific for melasma. This time frame was selected owing to the lack of a specific diagnosis code for melasma in the International Classification of Diseases, Ninth Revision. From this group, patients were selected at random for medical record-level validation. For each selected patient in the final validation cohort, demographic data, including age and sex, and diagnostic data were collected (Figure). The primary diagnostic criterion for melasma was medical record-level documentation of melasma as the favored diagnosis by the evaluating clinician. For patients meeting the primary criterion, the following secondary diagnostic criteria were used to estimate confidence in a diagnosis of melasma: (1) face and upper body distribution of hyperpigmentation, (2) exposure to hormone-related therapy before diagnosis, (3) history of pregnancy (excluded for male patients), and (4) diagnosis by a dermatologist (eMethods in the Supplement).3-6 Confidence in a diagnosis of melasma was calculated based on number of secondary criteria present (0-1, low confidence; 2, moderate confidence; and 3-4, high confidence; maximum confidence score for males was 3). After demonstrating more than 70% concordance on a smaller test cohort, trained personnel (J.Y., K.Y., and E.G.) validated medical record-level evidence of melasma for selected patients. The Mass General Brigham institutional review board approved this study and waived informed consent because aggregated electronic medical record data were used. The study followed the STROBE reporting guideline. Data were processed in RStudio, version 1.4.1103.
Of 5322 identified patients, 300 (5.6%) were selected at random for medical record-level validation (285 [95.0%] female; mean [SD] age at diagnosis, 48.4 [13.6] years). Most patients (291 [97.0%]) met the primary diagnostic criterion, yielding an overall positive predictive value of 97.0%. Most patients also met the secondary criteria of face and upper body distribution of hyperpigmentation (274 [91.3%]) and diagnosis by a dermatologist (252 [84.0%]). History of exposure to hormone-related therapy before diagnosis (148 [49.3%]) and pregnancy (168 [56.0%]) were less common. Confidence in a diagnosis of melasma was most commonly high (low: 31 patients [10.3%]; moderate: 61 [20.3%]; high: 208 [69.3%]) (Table). Therefore, our results suggest that our methods reliably identified patients with a diagnosis of melasma with a high degree of confidence of accurate diagnosis based on secondary criteria.
Overall, our results support the validity of using the ICD-10 code for melasma to identify patients with a diagnosis of melasma for future studies. Despite some variability in diagnostic confidence, most patients were ultimately classified as moderately or highly likely to have a true diagnosis of melasma. Limitations of this study included being a retrospective study of hospitals in a single region with a limited validation cohort size. Despite these limitations, our findings provide a framework for identifying cohorts to evaluate the clinical course and treatment of melasma.
Accepted for Publication: July 26, 2022.
Xem thêm : 101 Inspiring Mental Health Quotes
Published Online: November 9, 2022. doi:10.1001/jamadermatol.2022.3872
Corresponding Author: Nicholas Theodosakis, MD, PhD, Department of Dermatology, Massachusetts General Hospital, 55 Fruit St, Boston, MA 02114 (ntheodosakis@mgh.harvard.edu).
Author Contributions: Dr Theodosakis, Ms Yoon, Dr Mostaghimi, and Dr Semenov contributed equally to this study. Dr Theodosakis had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Theodosakis, Yoon, Mostaghimi, Semenov.
Acquisition, analysis, or interpretation of data: Theodosakis, Yoon, Young, Getachew, Mostaghimi.
Drafting of the manuscript: Theodosakis, Yoon.
Xem thêm : Ashwagandha: ¿Es útil para mejorar el estrés, la ansiedad y el sueño?
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Theodosakis, Yoon, Young, Getachew.
Administrative, technical, or material support: Theodosakis, Mostaghimi.
Supervision: Theodosakis, Mostaghimi, Semenov.
Conflict of Interest Disclosures: Dr Mostaghimi reported receiving personal fees from hims & hers, Digital Diagnostics, Pfizer, Eli Lilly and Company, AbbVie, Concert, Equillium, and Boehringer Ingelheim; having equity in hims & hers, Fig.1, Acom, and Seebe; having licensing with and/or receiving royalties from Pfizer, Concert, and Eli Lilly and Company; serving on medical advisory boards of hims & hers, Fig.1, and Digital Diagnostics; and participating in clinical trials conducted by Eli Lilly and Company and Concert outside the submitted work. Dr Semenov reported being an advisory board member and consultant for and receiving honoraria from Incyte Corporation, Castle Biosciences, Galderma, and Sanofi outside the submitted work. No other disclosures were reported.
Disclaimer: Arash Mostaghimi, MD, MPA, MPH, is Associate Editor for JAMA Dermatology but was not involved in any of the decisions regarding review of the manuscript or its acceptance.
Nguồn: https://blogtinhoc.edu.vn
Danh mục: Info
This post was last modified on Tháng mười một 22, 2024 6:21 chiều