Importance Case reports have suggested a link between human papillomavirus (HPV) vaccination and development of multiple sclerosis and other demyelinating diseases.
Objective To investigate if quadrivalent HPV (qHPV) vaccination is associated with an increased risk of multiple sclerosis and other demyelinating diseases.
Bạn đang xem: Quadrivalent HPV Vaccination and Risk of Multiple Sclerosis and Other Demyelinating Diseases of the Central Nervous System
Design, Setting, and Participants Using nationwide registers we identified a cohort of all females aged 10 years to 44 years in Denmark and Sweden, followed up from 2006 to 2013, information on qHPV vaccination, and data on incident diagnoses of multiple sclerosis and other demyelinating diseases. The primary analysis used a cohort design including vaccinated and unvaccinated study participants. A secondary analysis used a self-controlled case-series design including only cases. Both analyses used a 2-year risk period following vaccination.
Exposures Information on qHPV vaccination was obtained through the national vaccination and prescription registers.
Main Outcomes and Measures The primary outcomes were multiple sclerosis and a composite end point of other demyelinating diseases. Incidence rate ratios were estimated using Poisson regression, comparing rates of events in the 2-year risk periods following vaccination and in unvaccinated time periods.
Results The study included 3 983 824 females, among whom 789 082 received a total of 1 927 581 qHPV vaccine doses. During follow-up, 4322 multiple sclerosis cases and 3300 cases of other demyelinating diseases were identified, of which 73 and 90, respectively, occurred within the risk period. In the cohort analysis, there was no increased risk of multiple sclerosis (crude incidence rates, 6.12 events/100 000 person-years [95% CI, 4.86-7.69] and 21.54 events/100 000 person-years [95% CI, 20.90-22.20] for the vaccinated and unvaccinated periods; adjusted rate ratio, 0.90 [95% CI, 0.70-1.15]) or other demyelinating diseases (crude incidence rates, 7.54 events/100 000 person-years [95% CI, 6.13-9.27] and 16.14 events/100 000 person-years [95% CI, 15.58-16.71]; adjusted rate ratio, 1.00 [95% CI, 0.80-1.26]) associated with qHPV vaccination. Similarly, no increased risk was found using the self-controlled case-series design (multiple sclerosis: incidence ratio, 1.05 [95% CI, 0.79-1.38]; other demyelinating diseases: incidence ratio, 1.14 [95% CI, 0.88-1.47]).
Conclusions and Relevance In this study with nationwide coverage of 2 Scandinavian countries, qHPV vaccination was not associated with the development of multiple sclerosis or other demyelinating diseases. These findings do not support concerns about a causal relationship between qHPV vaccination and demyelinating diseases.
Since the licensure of the quadrivalent human papillomavirus (qHPV) vaccine in 2006 and the later licensure of the bivalent HPV (bHPV) vaccine, more than 175 million doses have been distributed worldwide.1 The introduction of large-scale vaccination in a new target group—girls and young women—has been accompanied by a number of safety concerns, with the potential to undermine public confidence in the new vaccines.2-5 One concern is the development of multiple sclerosis, which has been fuelled by social and news media reports of cases occurring after HPV vaccination1 together with an increasing number of case reports published in the medical literature describing vaccinees who developed multiple sclerosis as well as other demyelinating diseases, including optic neuritis, transverse myelitis, acute disseminated encephalomyelitis, and neuromyelitis optica.6-10 These concerns have been highlighted by the Global Advisory Committee on Vaccine Safety of the World Health Organization and were considered in a safety assessment conducted by the European Medicines Agency.1,11 Potential mechanisms by which vaccines could induce autoimmune diseases have been suggested to include molecular mimicry and bystander activation.12 It is, however, not known if the occurrence of these conditions after HPV vaccination merely reflects the background rates in girls and young women or represents a true increased risk. The few published analytical studies investigating the association between HPV vaccines and multiple sclerosis and other demyelinating diseases, while largely finding no significantly increased risks, have had limited statistical power to exclude even moderate to large increased risks of these conditions.13-15
We conducted a cohort study of all Danish and Swedish girls and women aged 10 years to 44 years on the basis of nationwide registers and investigated the risk of multiple sclerosis and other demyelinating diseases of the central nervous system following qHPV vaccination.
We established a combined cohort comprising all Danish girls and women aged 10 years to 44 years during the period from October 1, 2006, to July 1, 2013, and all Swedish girls and women aged 10 years to 44 years during the period from October 1, 2006, until December 31, 2012 (because data were not available for the Swedish cohort beyond this date), respectively, using data from the Civil Registration System in Denmark and Statistics Sweden (registers described in the eAppendix in the Supplement).16 The unique personal identification number given to every resident in Denmark and Sweden enabled the individual-level linkage of our cohort with nationwide health and demographic registers containing information on vaccination and demyelinating diseases.17 Participant informed consent was not required. The regional ethics committee in Stockholm, Sweden, approved the study. No ethics approval is needed for register studies in Denmark. The study was approved by the Danish Data Protection Agency.
The qHPV vaccine (Gardasil; Sanofi Pasteur MSD SNC [in the United States: Merck]) was licensed in Europe in September 2006 and recommended as a 3-dose schedule throughout the study period, with the second and third doses given 2 and 6 months, respectively, after the first dose.18,19 In Denmark, the qHPV vaccine has been included in the national vaccination program since 2009 for 12-year-old girls, with catch-up vaccination of girls aged 13 years to 15 years from October 2008 and of young women aged 20 to 27 years from August 2012. In Sweden, the qHPV vaccine has been subsidized since May 2007 and was subsequently implemented in the national vaccination program for 10- to 12-year-old girls in January 2012, together with catch-up vaccination of 13- to 17-year-old girls. Because the use of the bHPV vaccine is uncommon in Denmark and Sweden (given to <1% of all vaccinees),13 the association between demyelinating diseases and the bHPV vaccine was not analyzed.
Data on vaccination with the qHPV vaccine in Denmark were obtained via the Childhood Vaccination Database at Statens Serum Institut, Denmark, which includes all vaccinations given through the national vaccination program.20 Furthermore, the qHPV vaccine was also available by prescription (since its introduction in 2006); additional data were therefore obtained from the National Prescription Registry (Anatomical Therapeutic Chemical code J07BM01).21 Similarly, information on qHPV vaccination in Sweden was obtained from Svevac22 (the national HPV vaccination register) and the Swedish Prescribed Drug Register.23 Some participants had more than 3 qHPV vaccinations recorded, most often participants with recordings from both prescription and vaccination databases. Because data differed for the 2 countries, we applied 2 different algorithms to harmonize data. For the Danish data, the first recording was discarded when vaccinations were closely spaced (within 7 days). For the Swedish data, an algorithm discarded any prescription dispensed within 14 days before the vaccine date.
Using information from nationwide patient registers in both countries, which include data on physician-assigned diagnoses from hospital inpatient and outpatient departments, we identified predefined outcomes by International Statistical Classification of Diseases, Tenth Revision (ICD-10) codes.24,25 We evaluated 2 outcomes. First, since concern has mainly been directed at multiple sclerosis, we included a main outcome defined as an incident diagnosis of multiple sclerosis (ICD-10 code G35).1 Second, since reports have also included other central demyelinating diseases,6-10 we created a composite outcome comprising the first incident diagnosis of the following demyelinating diseases: optic neuritis (ICD-10 code H46), neuromyelitis optica (ICD-10 code G360), transverse myelitis (ICD-10 code G373), acute disseminated encephalomyelitis (ICD-10 codes G040, G378), and other central demyelinating diseases (ICD-10 codes G368, G369, G379). A similar composite end point was used in a previous study investigating the association between hepatitis B vaccine and central demyelinating diseases.26 The index event date was defined as the date of diagnosis.
Our primary analysis was a cohort analysis, in which follow-up was from October 1, 2006, or age 10 years, whichever came last, until death, emigration, age 45 years, receipt of the bHPV vaccine (available for the Danish cohort only), a diagnosis of demyelinating disease, or end of follow-up (December 31, 2012, for the Swedish data; July 1, 2013, for the Danish data), whichever came first. Both outcomes were treated as separate analyses, and for each specific analysis only girls and women free of the outcome before entry were eligible for inclusion in the cohort. The resulting person-years were aggregated with counts of outcome events according to qHPV vaccination dates and analyzed using Poisson regression. Each participant could contribute both unvaccinated and vaccinated person-time.
Xem thêm : How to build a hiker’s first aid kit
Because of the insidious onset of the diseases studied and often protracted diagnostic workup, we considered a 2-year (730 days) risk period following the latest qHPV vaccination, similar to the risk period used in a previous study investigating the association between hepatitis B vaccine and multiple sclerosis.27 The 2-year risk period was reentered after each dose of qHPV vaccine; assuming vaccination according to the recommended schedule at 0, 2, and 6 months, each participant could contribute up to 2½ years of follow-up in the risk period. Person-time after the vaccination risk periods was not discarded, contributed information on adjustment variables, and was also used for sensitivity analyses; however, once exposed to the qHPV vaccine, participants could not reenter the unexposed group in the cohort analysis. Incidence rate ratios (RRs) were estimated by comparing rates of events in the 2-year risk periods following vaccination and in unvaccinated time periods. The cohort analysis was adjusted for calendar year, age (2-year intervals), and country.
To evaluate the potential for unmeasured time-independent confounding in our main analysis, we conducted a self-controlled case-series analysis within our cohort.28,29 The self-controlled case-series method compares person-time within cases only. A benefit of within-person comparisons is that time-independent factors such as socioeconomic status, general health, and genetic predisposition cannot confound the estimates. We used the cases from our cohort and the same follow-up, except that when cases developed, follow-up was not terminated but continued until any of the other conditions for ending follow-up in the main cohort analysis were met. In the self-controlled case-series analyses, cases occurring after the 2-year risk period were included in the modeling and therefore the number of cases differed between the self-controlled case-series analysis and the cohort analysis. A conditional Poisson model was used to derive incidence ratios (IRs) by comparing the rate of events during the 2-year risk period with that during all other observed time periods (unvaccinated time and, when applicable, time 2 years after vaccination) for each case. The self-controlled case-series analysis was adjusted for age in 2-year categories.
A number of additional cohort analyses were conducted to evaluate the robustness of the results. First, we explored 4 alternative risk windows: 0-179, 180-364, 365-729, and more than 729 days after vaccination. Second, because the risk might differ for girls and young women compared with mid-adult women, we conducted analyses stratified by age (10-29 years and 30-44 years). Third, because the baseline incidence of demyelinating diseases may differ between Denmark and Sweden, we conducted analyses stratified by country. Fourth, if becoming a case contraindicates or delays vaccination in a significant number of participants, the self-controlled case-series results can be biased. To address this concern we conducted 2 additional analyses—one excluding a 30-day prevaccination period and one including only vaccinated cases.
A 2-sided 95% CI that did not overlap 1.0 and P < .05 were considered statistically significant. All statistical calculations were performed using SAS version 9.4 (SAS Institute Inc).
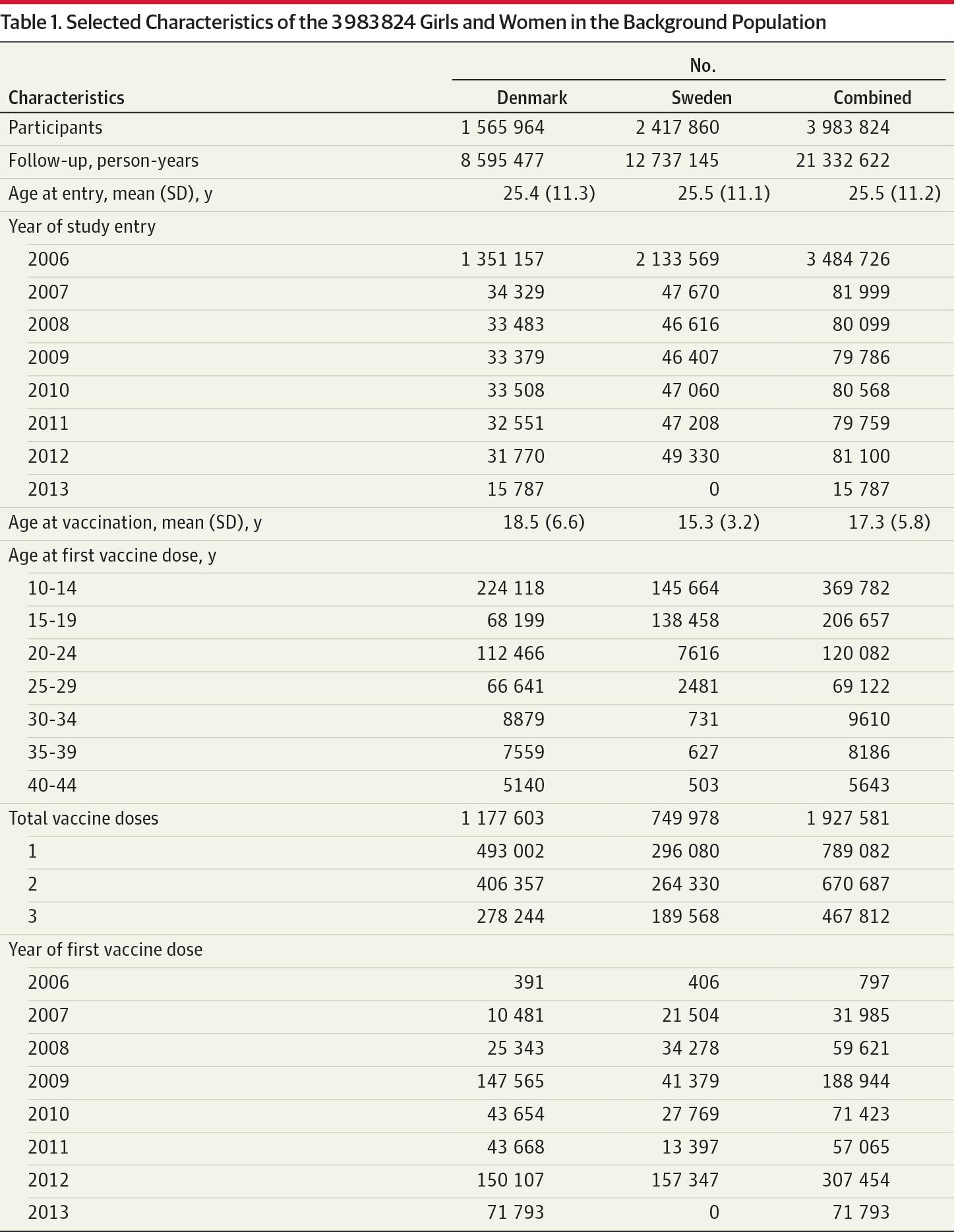
A total of 3 983 824 girls and women were eligible for inclusion in the cohort. Of these, a total of 789 082 were vaccinated during the study period, with a total of 1 927 581 qHPV vaccine doses; 670 687 received at least 2 doses and 467 812 received 3 doses. The girls and women were followed up for a total of 21 332 622 person-years, mean age at entry was approximately 25.5 years both for Denmark and Sweden, mean age at vaccination was slightly higher in Denmark (18.5 years) compared with Sweden (15.3 years), and the temporal trends of vaccination reflected the trends in the national vaccination programs in Denmark and Sweden, respectively (Table 1). Following the exclusion of 5553 individuals because of prevalent multiple sclerosis, 3 978 271 girls and women were included in the cohort analysis of multiple sclerosis. Correspondingly, 3 980 716 were included in the analysis of other demyelinating disease after the exclusion of 3108 individuals with a history of such diseases. The Figure depicts the inclusion of girls and women in the cohort analysis and self-controlled case-series analysis.
We observed 73 cases of multiple sclerosis and 90 cases of other demyelinating disease during vaccinated risk periods in the cohort analysis and 4208 cases of multiple sclerosis and 3154 cases of other demyelinating disease during unvaccinated risk periods. In the cohort analysis, there was no increased risk of multiple sclerosis (crude incidence rates, 6.12 events/100 000 person-years [95% CI, 4.86-7.69] for the vaccinated period and 21.54 events/100 000 person-years [95% CI, 20.90-22.20] for the unvaccinated period; adjusted RR, 0.90 [95% CI, 0.70-1.15]) (Table 2) or the composite outcome of other demyelinating disease (crude incidence rates, 7.54 events/100 000 person-years [95% CI, 6.13-9.27] for the vaccinated period and 16.14 events/100 000 person-years [95% CI, 15.58-16.71] for the unvaccinated period; adjusted RR, 1.00 [95% CI, 0.80-1.26]) (Table 2).
There were 4322 cases of multiple sclerosis (339 vaccinated) and 3300 cases of other demyelinating diseases (370 vaccinated) included in the self-controlled case-series analysis. Consistent with the cohort analysis, the self-controlled case-series analysis showed no increased risk of multiple sclerosis (IR, 1.05 [95% CI, 0.79-1.38]) or other demyelinating diseases (IR, 1.14 [95% CI, 0.88-1.47]). Excluding a prevaccination risk period of 30 days from the unexposed group did not significantly change the results of the self-controlled case-series analysis (IR for multiple sclerosis, 1.12 [95% CI, 0.84-1.49] ; IR for other demyelinating diseases, 1.16 [95% CI, 0.89-1.51]). Similarly, using only exposed cases did not significantly change the results of the self-controlled case-series analysis (IR for multiple sclerosis, 1.07 [95% CI, 0.80-1.43]; IR for other demyelinating diseases, 1.14 [95% CI, 0.88-1.48]).
Analyses by country, by age, and with different risk windows corroborated the results from the cohort analyses of no increased risk (Table 2).
In all girls and women aged 10 years to 44 years in Denmark and Sweden, we observed no increased risk of multiple sclerosis or other demyelinating diseases following qHPV vaccination. These findings were confirmed in self-controlled case-series analyses and were further corroborated by additional sensitivity analyses. Given the upper limits of the confidence intervals, the study can exclude a potential 16% increased risk of multiple sclerosis and a 27% increased risk of other central demyelinating diseases.
Our study has a number of strengths. First, it was based on individual and complete records of vaccination and outcome diagnoses ascertained prospectively and independently in well-defined geographical areas. Correspondingly, concern over recall and ascertainment bias has been minimized. Second, by combining data from Sweden and Denmark, countries with comparable public health systems and registers, the study had statistical power to exclude meaningful increases in risk, which is reflected in the narrow 95% CIs. Third, the nationwide nature of our study allows for a high degree of generalizability of our results; this includes rates of demyelinating diseases based on true background rates.
Our study had a number of limitations. First, information on ethnicity, socioeconomic status, lifestyle factors, and family history was not available, and potential confounding was therefore a possibility in the cohort analysis. However, the self-controlled case-series analysis, which implicitly controls for time-independent confounders, suggested that bias attributable to such unmeasured confounders was minimal and confirmed the results from the cohort analysis of no risk of multiple sclerosis and other demyelinating diseases following qHPV vaccination. Second, our case definition relied on ICD-10 codes registered in national patient registries. In a validation study of the Danish National Patient Registry, the predictive value of the diagnosis of multiple sclerosis was 95% and completeness of registration was 93%.30 Although a similar disease-specific validation of the Swedish Patient Register has not been performed, Sweden and Denmark have very similar health care systems and any differences are likely to be small. Furthermore, analyses by country showed no increased risk of multiple sclerosis or other demyelinating diseases in either Denmark or Sweden alone. The outcomes in the composite end point have not been validated, and registration could be less complete than that for multiple sclerosis.
Third, previous vaccine safety studies have described a so-called unmasking phenomenon, which refers to the fact that vaccinations provide the opportunity to evaluate symptoms that would have otherwise gone unnoticed, resulting in bias toward an association.31 This is applicable to diseases, such as multiple sclerosis, presenting with obscure symptoms, while less applicable to diseases with relatively prominent and well-recognized symptoms. However, our null results are unlikely to reflect such a bias. Fourth, our study used date of diagnosis instead of date of disease onset, which is not available through the hospital registers. If onset and diagnosis did not occur in the same exposure period, cases would have been misclassified. Random misclassification would bias results toward no association. In the event of differential misclassification, eg, if symptoms contraindicate vaccination, results would be biased toward a protective association. To account for any delay from onset to diagnosis we chose a 2-year risk period; in a US study the diagnostic delay has been estimated to be less than 1 year in more than 50% of cases occurring after 2000.32 Furthermore, analyses subdividing postvaccination time into a number of different risk intervals did not reveal any temporal patterns suggestive of bias or the presence of risk increases masked by the 2-year risk period. Fifth, if becoming a case contraindicates or delays vaccination in a significant number of participants, the self-controlled case-series results can be biased. Supplementary self-controlled case-series analyses that addressed this concern by either excluding prevaccination time or including only vaccinated cases supported the primary self-controlled case-series analysis.
Comparing crude rates to adjusted RRs, there was substantial influence from adjustment. This derives from the fact that both vaccination and outcomes are age dependent. The vaccinated group was younger on average than the unvaccinated group, and since the background incidence of demyelinating diseases peaks later among the included age groups, the crude rates were higher in the unvaccinated group compared with the vaccinated group.
Since demyelinating diseases are relatively rare, previous controlled observational studies have been unable to reliably confirm or refute a potential association with qHPV vaccination. A cohort study in California, including 189 629 girls and women with receipt of the qHPV vaccine, studied the potential association with central demyelinating diseases. Although that study found no significant associations, the precision of the estimates did not allow the exclusion of less than approximately 3-fold increases in the risk of multiple sclerosis (RR, 1.37 [95% CI, 0.74-3.20]) and optic neuritis (RR, 1.45 [95% CI, 1.00-2.91]) or of less than an approximately 2-fold increased risk of other demyelinating diseases (RR, 0.71 [95% CI, 0.38-2.13]).14 In a previous study, we investigated the risk of a range of disease outcomes in approximately 300 000 qHPV-vaccinated girls; our previous study used the same databases but had a shorter study period, was restricted to girls younger than 18 years, and was not powered to investigate multiple sclerosis or most other demyelinating diseases (apart from optic neuritis; RR, 0.67 [95% CI, 0.27-1.64]).13 A case-control study in France investigated the risk of a combined end point of central demyelinating diseases and multiple sclerosis. Based on only 4 vaccine-exposed cases, a significant decreased risk of central demyelinating diseases was observed (adjusted odds ratio, 0.3 [95% CI, 0.1-0.9]). The estimate of a 70% lower risk of central demyelinating diseases may be the effect of selection bias, because cases were selected from a hospital-based population while controls were selected from a population of patients from general practitioners.15,33
Our study adds to the body of data that support a favorable overall safety profile of the qHPV vaccine and expands on this knowledge by providing comprehensive analyses of multiple sclerosis and other demyelinating diseases.34 The size of the study (including almost 4 million girls and women, among whom approximately 800 000 were vaccinated) and the use of nationwide registry data of unselected populations from Denmark and Sweden allowed adequately powered analyses that are likely generalizable.
Xem thêm : Can My Parents Force Me to Go to Therapy or Rehab?
In this study with nationwide coverage of 2 Scandinavian countries, qHPV vaccination, among girls and women, was not associated with the development of multiple sclerosis or other demyelinating diseases. These findings do not support concerns about a causal relationship between qHPV vaccination and demyelinating diseases.
Corresponding Author: Nikolai Madrid Scheller, MB, Department of Epidemiology Research, Statens Serum Institut, Artillerivej 5, 2300 Copenhagen S, Denmark (nims@ssi.dk).
Author Contributions: Mr Scheller had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Scheller, Svanström, Pasternak, Arnheim-Dahlström, Fink, Hviid.
Acquisition, analysis, or interpretation of data: Scheller, Svanström, Pasternak, Arnheim-Dahlström, Sundström, Hviid.
Drafting of the manuscript: Scheller.
Critical revision of the manuscript for important intellectual content: Svanström, Pasternak, Arnheim-Dahlström, Sundström, Fink, Hviid.
Statistical analysis: Scheller, Svanström.
Obtained funding: Scheller, Pasternak, Arnheim-Dahlström, Hviid.
Administrative, technical, or material support: Fink.
Study supervision: Svanström, Pasternak, Hviid.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Arnheim-Dahlström reported receiving grants from MSD Sanofi Pasteur, Merck Sharp & Dohme, and GlaxoSmithKline for other studies. Dr Fink reported receiving travel support from Biogen Idec for other studies. Dr Hviid reported receiving a grant from the Novo Nordisk Foundation. No other authors reported disclosures.
Funding/Support: The Swedish Foundation for Strategic Research, Novo Nordisk Foundation, and The Danish Medical Research Council funded the study.
Role of the Funders/Sponsors: The study funders/sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: We thank Eva Herweijer, MSc (Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden), for database administration and the Public Health Agency of Sweden for contributing with human papillomavirus vaccine data from the Svevac register. Ms Herweijer received no compensation for her contributions.
Nguồn: https://blogtinhoc.edu.vn
Danh mục: Info
This post was last modified on Tháng mười một 25, 2024 6:11 chiều