Alexander Ladenheim, M.D., Pathology ResidentMelanie Rilloraza, C.L.S. (ASCP), Microbiology SupervisorNam K. Tran, Ph.D., Director of Clinical ChemistryAnna Romanelli, Ph.D., Medical Director of Clinical Microbiology Laboratory
Topics
- Introduction
- Expired Collection Products
- Anaerobic Cultures
- Lukens Traps for Mucus/Aspirate Specimen Collection
- Surveillance Cultures (MRSA and C. difficile)
- Other Cultures Requiring Special Media
- References
Introduction
The culturing of microorganisms remains the mainstay of laboratory testing for infectious disease, even in an age of rapid and more cost-effective molecular testing. For cultures to be reliable, special attention needs to be paid to the collection, transport, and processing of these specimens. The goal of microbiologic culture is the preservation of viable clinically relevant organisms, specifically ones which are likely to be pathogenic. A negative culture result is less helpful for ruling out disease since there is always possibility that organisms were rendered nonviable by incorrect collection or handling. Similarly, positive cultures can be confounded by the presence of contaminant microorganisms and rendered extremely hard to interpret.
Bạn đang xem: Specimen Collection Practices for Microbiologic Culture
Specimen collection is a team effort and involves the clinical team, couriers, and laboratory personnel, each of whom can have a drastic influence on the quality of the final result. The clinical team in many ways sets the parameters of testing by forming a differential diagnosis and choosing many of the basic conditions: the method of collection, an appropriate site, and sampling. The laboratory, in turn, makes a commitment to providing education and resources for appropriate collection and to promptly and correctly process received specimens. Furthermore, if a sample is compromised or otherwise unlikely to provide useful diagnostic information, the lab has a responsibility to reach out to the clinical team to educate and coordinate the collection of an optimal sample. This is perhaps just as important as rapidly and accurately reporting results but easily overlooked in the hustle and bustle of clinical care.
What follows is a discussion of several common problems in specimen collection:
The “sniff test”: Expired collection products
Expired media cannot be relied upon for specimen collection. It is not uncommon for expired blood culture vials and swab kits to accumulate in the myriad supply closets of the hospital, but these should be identified and replaced. The unit manager, charge nurse, or other person responsible for inventory management can obtain replacements with a call to UCD Supply Chain Distribution or Microbiology.
The use of expired media is unacceptable both from a laboratory accreditation perspective (by the requirements of the College of American Pathologists) and from a patient care perspective; it leads to the risk of both false negative and false positive results. Collection media is a specially designed, pH balanced, sterile blend of food for microorganisms and myriad other components1 which can include:
- Reducing agents to promote the growth of anaerobic bacteria (inactivated by oxygen)
- Selective agents to promote the growth of particular microorganisms
- Resins and charcoal to neutralize antibiotics and promote growth
Depending on the specific type of collection media, some of these components are more labile than others, which can result in a shorter shelf-life. Some specialized collection media (such as thioglycolate broth) are so labile that indicator dyes are added to show when a vial of medium is no longer usable.2 In addition, the majority of collection media are not subject to quality control testing by users (i.e., the laboratory) and are considered exempt under the National Committee for Clinical Laboratory Standards (M22-A3). As such, the laboratory relies on manufacturer parameters with respect to expiration dates and storage conditions.
Questions & Answers (Q&A)
Q: How do I know if my blood culture media is expired?A: The expiration date is on the bottle near the measuring guides (Figure 1).
Figure 1. BD Bactec blood culture bottle with expiration date (photo: UC Davis Health microbiology)
Q: What should I do if I have expired media?A: Call and ask for these items to be re-stocked. Most items are either stocked by UCD Supply Chain Distribution (3-4040) or your unit may have a designated staff person responsible for inventory and ordering supplies.
Q: What if I am part of a PCN (primary care network) location?A: Most PCNs order their own supplies. Questions regarding collection kits for PCNs should be directed to Laboratory Client Services 916-734-7373.
Culture for anaerobic organisms: no swabs allowed!
Xem thêm : Vision Care Expert Discusses the Challenges of Living with Pterygium
Anaerobic bacteria survive in oxygen poor regions of the body and make up a large percentage of the commensal, normal flora. As such, most anaerobic infections are endogenous; they result from damage to tissue and invasion of otherwise sterile sites.
Certain body sites are known to have a high propensity of anaerobic infection:
Head and neckDental abscessAbdomenIntraabdominal infections/abscessesChronic otitis mediaClostridioides difficile colitisBrain abscessUrogenitalEndometritisSkin/soft tissueBite woundsPelvic inflammatory diseaseNecrotizing fasciitisPulmonaryAspiration pneumoniaPerirectal abscess
At many of these sites, a mix of flora (including aerobic/facultative anaerobes) are present. As such, collection must be performed carefully to avoid contaminated cultures which are difficult to interpret. The sites listed above tend to be deep, and cultures are often obtained operatively. The best specimens are aspirates of abscesses or excisional biopsies (from the wound edge).3
Superficial swabs or swabs of pus (Figure 2, left) are almost never appropriate due to the high risk of a contaminated specimen. Additionally, swabs present other problems. Many are made of cotton fibers and are porous. As such, specimen tends to dry onto the swab and is poorly released into the transport medium in the vial. Further, the swabs contain fatty acids which inhibit bacterial growth of anaerobic organisms which naturally tend to be fastidious and difficult to recover in culture. Finally, the transport media in swab vials are not optimal for recovery.3 Although the manufacturers of certain non-cotton swabs (such as the Copan eSwab) claim to recover anaerobes,4 the anaerobic culture method at UCDMC has not been validated using these, and thus samples obtained by swab cannot be reported out.
Instead, needle aspirates or tissue biopsies should be placed into anaerobic transport medium (ATM), which is specially designed to exclude oxygen and preserve viable anaerobes (Figure 2, right). Specimens should be transported to the lab at room temperature (oxygen is able to diffuse into the liquid medium more easily at low temperatures), 3 ideally within 3 hours of collection; specimens older than 24 hours will not be accepted. Please note that susceptibility testing in suspected anaerobic infection requires approval by Infectious Disease.
Q&A
Q: How can I obtain anaerobic transport medium for my biopsy/aspirate?A: Most lab collection supplies are either stocked by UCD Supply Chain Distribution (3-4040), or your unit may have a designated staff person responsible for inventory and ordering supplies. Anaerobic transport medium is Lawson Item #100666.
Q: What if I am part of a PCN (primary care network) location?A: Most PCNs order their own supplies. Questions regarding collection kits for PCNs should be directed to Laboratory Client Services 916-734-7373.
The curse of the leaky Lukens trap
The Lukens trap is a sterile container placed in-line with the suction catheter for the collection of endotracheal aspirates or bronchoalveolar lavage fluid. Its 2-port design keeps mucus/fluid out of the vacuum line of the evacuation system. After collection, the suction adaptor cap should be removed from the Lukens trap and exchanged for a sterile transport cap (Figure 3), which should be screwed on securely, and the trap should be placed in secondary containment (a biohazard bag).
Although in the past it has been common practice to simply close up the trap with tubing, do not do this! Lukens traps are famous for leaking, as the tubing easily becomes dislodged during transport. Although the trap is in secondary containment, the specimen is also usually transported on ice. Melting ice cannot easily be distinguished from a leaking specimen. As a result, traps which appear to be leaking into their secondary containment are usually rejected, both because of the possibility of a contaminated specimen and for the safety of laboratory personnel processing the specimen.
The current outbreak of COVID-19 is a reminder that safety is paramount when it comes to specimens containing unknown infectious agents. Leaking specimens pose a risk to personnel at all levels of specimen handling, including the clinical team, couriers, and laboratory personnel.
A number of clinical services currently utilize Lukens trap kits which do not contain screw-top transport caps. The laboratory has worked with UCD Supply Chain Distribution to replace these older kits; new kits which do contain transport caps are now being distributed.
Q&A
Xem thêm : Los mejores consejos para curar el ombligo del recién nacido
Q: My department still uses Lukens trap kits without transport caps. How do I get them replaced?A: The new kits are available as of 4/7/2020 through UCD Supply Chain Distribution (3-4040). Call and ask for them to be re-stocked, and the new kits will come with transport caps.
Surveillance cultures: choose the right medium!
Patients at UCDMC who are newly admitted to the inpatient services are now routinely screened for colonization by methicillin-resistant Staphylococcus aureus (MRSA) and Clostridioides difficile to reduce the risk of hospital acquired infections. The testing is noninvasive and performed by swab of the anterior nares (MRSA) and anus (C. difficile). Testing is rapidly performed and clinically actionable, allowing isolation of patients with positive results. Although the efficacy of universal MRSA screening remains controversial,5 California state law requires, at minimum, targeted screening.6 Some studies (such as data from the VA MRSA Prevention Initiative) have demonstrated that universal active surveillance can decrease hospital acquired MRSA infections by as much as 80%.7 Similarly, admission screening for C. difficile carriage may reduce hospital acquired C. difficile infection by up to 50%.8,9
Collection of MRSA and C. difficile screening specimens is performed with 2 different swab kits. The MRSA test is performed using the Copan eSwab. The C. difficile test is performed using a BD BBL CultureSwab placed in a special, nonnutritive medium (Stuart Transport Medium).
There are several reasons for using different swabs. First and foremost, these tests are laboratory developed tests, meaning the test method was developed here at UCDMC, and it must be rigorously outlined and validated in a process which can take months to years. As such, for results to be reportable, the test must be carried out from collection to processing in a manner consistent with the validated method.
Second, there have been several incidences of accidental sample mix-up, no doubt because both tests are being performed early in admission and use swabs. However, sample mix-up can lead to false negative test results. This type of culture is not a routine culture. The MRSA test utilizes selective media and indicator dyes which change color in the presence of colonies after plating and incubation. The C. difficile test is a PCR-based test. Therefore, in the lab, if a swab used to collect an anterior nares specimen is mislabeled as C. difficile and run on PCR, it will generate a potentially false negative result.
The laboratory is working with nursing staff to improve specimen collection practices. Job aids depicting the appropriate swab kit for each test have been deployed, and the laboratory is working with IT to change the specimen labels for each test so that they prominently display the type of swab kit which should be used. In the end, if a specimen is accidentally collected on the wrong swab or mislabeled, it cannot be changed after the lab receives it. Instead, the best practice is to recollect the specimen; it is relatively noninvasive, quick, and safer for the patient.
Cultures requiring special media: don’t you forget about me!
Cultures for fastidious/uncommon organisms require special care. Collection of these specimens into generic or incorrect media may result in several problems making recovery of the suspected target organism poor or impossible:
- Poor growth conditions: special nutritional/environmental requirements
- Overgrowth of off-target bacteria/contaminants
- Dilution by collection media
A negative test result in the context of a suboptimal collection raises the specter of a false negative and provides no useful clinical information. The following cultures in particular require special media:
- Acid fast bacilli (Mycobacteria) culture
- Tissue/fluid culture: as much volume as possible, collected in a sterile container
- Swabs are unacceptable: too little volume
- Blood culture: green top tube (sodium or lithium heparin, light or dark green)
- Routine blood culture bottles are unacceptable
- Tissue/fluid culture: as much volume as possible, collected in a sterile container
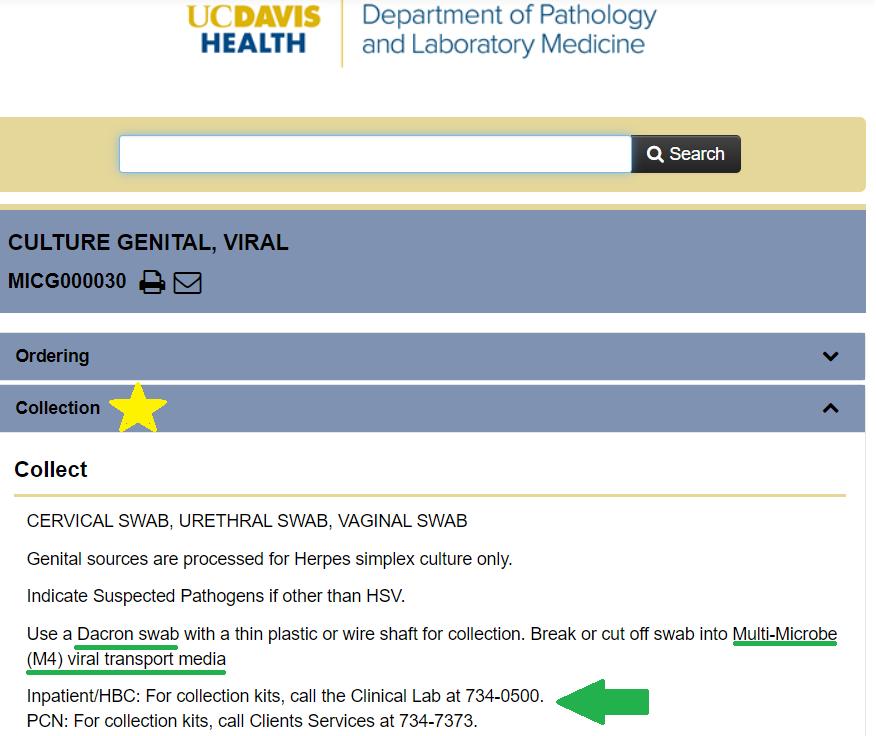
- Viral culture
- Universal transport media (UTM) or viral transport media (M4)
- Specimens should be transported on ice
- Use Dacron/polyester swabs, fully immersed in medium
- Cotton swabs/toothpicks/dry swabs will not work for reasons described above in the section on anaerobic cultures
- Universal transport media (UTM) or viral transport media (M4)
- Fungal blood cultures
- A special isolator tube is used
- These cultures are for the detection of unusual pathogenic fungi (such as Histoplasma, Cryptococcus, Blastomyces, and Malassezia).
- Remember: fungal blood cultures are not for the detection of common fungi like most Candida and Aspergillus. These organisms grow well in routine, automated blood cultures which have faster turnaround times, rapid isolation/susceptibility testing, and less impact on laboratory workflow.
- Please see this previous Laboratory Best Practices blog article for more information.
Q&A
Q: How do I find out which tests require special media?A: Check out the UC Davis Test Menu (https://www.testmenu.com/ucdavis, Figure 5), or call the clinical laboratory at (916) 734-0500.
Figure 5. Specimen collection information can be found online at the UC Davis Health Test Menu (annotated screenshot, https://www.testmenu.com/ucdavis, accessed March 5, 2020)
Q: How do I obtain media for these specialized cultures?A: Check out the UC Davis Test Menu (https://www.testmenu.com/ucdavis), or call the clinical laboratory at (916) 734-0500.
Q: What if I am part of a PCN (primary care network) location?A: Most PCNs order their own supplies. Questions regarding collection kits for PCNs should be directed to Laboratory Client Services 916-734-7373.
References
- Ulisse S, Peccio A, Orsini G, Di Emidio B. “A study of the shelf-life of critical culture media.” Veterinaria Italiana 2006;42(3):237-247.
- Sutton S. “Quality control of microbiological culture media.” Pharmaceutical Microbiology Forum 2006;12(1):2-5. Available at: http://www.microbiologyforum.org/pmf_newsletters.asp. Accessed March 17, 2020.
- Nagy E, Boyanova L, Justesen US. “How to isolate, identify and determine antimicrobial susceptibility of anaerobic bacteria in routine laboratories.” Clin Microbiol Infect. 2018 Nov;24(11):1139-1148. doi: 10.1016/j.cmi.2018.02.008. Epub 2018 Feb 17.
- Copan USA. “eSwab: Product Insert.” Last updated February 2016. Available at: https://www.copanusa.com/wp-content/uploads/2019/07/ESwab-Package-Insert_HPC030_eSwab_copoliestere_Rev00_Date2016.02.pdf. Accessed March 18, 2020.
- Calfee DP, Salgado CD, Milstone AM, Harris AD, Kuhar DT, Moody J, Aureden K, Huang SS, Maragakis JL, Yokoe DS. “Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute care hospitals: 2014 update.” Infection Control and Hospital Epidemiology 2014;35(2):108-132.
- California Health and Safety Code 1255.8
- Evans ME, Kralovic SM, Simbartl LA, Jain R, Roselle GA. “Eight years of decreased methicillin-resistant Staphylococcus aureus health care-associated infections associated with a Veterans Affairs prevention initiative.” AJIC 2017;45(1):13-16.
- Longtin Y, Paquet-Bolduc B, Gilca R, Garenc C, Fortin E, Longtin J, Trottier S, Gervais P, Roussy JF, Lévesque S, Ben-David D, Cloutier I, Loo VG. “Effect of Detecting and Isolating Clostridium difficile Carriers at Hospital Admission on the Incidence of C difficile Infections: A Quasi-Experimental Controlled Study.” JAMA Intern Med 2016 Jun 1;176(6):796-804. doi: 10.1001/jamainternmed.2016.0177.
- Peterson LR, O’Grady S, Keegan M, et al. “Reduced Clostridioides difficile infection in a pragmatic stepped-wedge initiative using admission surveillance to detect colonization.” PLoS One. 2020;15(3):e0230475. doi:10.1371/journal.pone.0230475.
Nguồn: https://blogtinhoc.edu.vn
Danh mục: Info
This post was last modified on Tháng mười một 23, 2024 3:41 chiều