Objective The β2-transferrin assay is a specific method to identify cerebrospinal fluid (CSF). Hitherto, this test has not been widely used for the routine screening of patients with suspected CSF leakage. The purpose of this study was to investigate the clinical relevance of the identification of β2-transferrin by comparing the test results with other diagnostic measures and intraoperative findings.
Design Case series.
Bạn đang xem: Diagnostic Relevance of β2-Transferrin for the Detection of Cerebrospinal Fluid Fistulas
Patients Retrospective analysis of 182 patients tested once or multiple times for β2-transferrin.
Main Outcome Measures Information was obtained regarding different diagnostic procedures applied to diagnose CSF leakage. The effectiveness of those diagnostic measures was compared.
Results The main indication to test for β2-transferrin was posttraumatic rhinorrhea (25%), followed by spontaneous (22%) and postsurgical (22%) rhinorrhea. In 35 of 205 cases, β2-transferrin was detected in the tested specimens. Thirteen of these required surgical intervention for treatment of the CSF fistula, and the leakage site was identified in all of them. Taking all results into consideration, the highest correlation was observed between the β2-transferrin assay, intrathecal fluorescein application, and surgical exploration.
Conclusions The β2-transferrin assay is a reliable method for confirming suspected CSF and should be used as a primary screening method in all patients with suspected CSF leakage. Although less invasive, the β2-transferrin assay almost matches the high sensitivity achieved by exploratory surgery and intrathecal application of fluorescein. However, the possibility of bias should be carefully considered, and in particular, negative results should be critically compared with clinical symptoms and with results from other diagnostic procedures.
Cerebrospinal fluid (CSF) fistulas can become a life-threatening condition. These occur following a penetrating injury of the leptomeninges, dura, and bone, resulting in an extracranial discharge of CSF and providing an entry for pathogens into the subarachnoid space, potentially leading to meningitis. Therefore, early diagnosis of a CSF leak in patients with rhinorrhea or otorrhea of uncertain origin is essential.
In patients with profuse postsurgical or posttraumatic otorrhea or rhinorrhea, the diagnosis is obvious and thus only needs to be confirmed. However, in patients with minimal or intermittent CSF discharge, the diagnosis and the localization of the leak may present a challenge. Different diagnostic procedures are available and are commonly used in patients with suspected CSF leakage.1Computed tomographic (CT) scanning can localize a fracture line, a bony dehiscence, or intracranial air, but fails to demonstrate persistent violation of the leptomeninges with CSF leakage. It is therefore difficult to base an indication for invasive treatment options such as surgical exploration or lumbar drainage on indirect evidence of the presence of a CSF fistula as is found on a CT scan. To obtain more reliable indications or even direct demonstration of the CSF leakage, invasive methods seem unavoidable. Computed tomographic imaging following intrathecal application of radiopaque agents can confirm the diagnosis and may also localize the CSF leak. However, invasive methods are inconvenient for the patient, and contrast CT scanning or scintigraphy with radioactive tracers are potentially dangerous.2,3 Furthermore, these methods can only confirm the diagnosis when leakage occurs during the time of examination but may fail in cases of intermittent CSF leakage. Surgical exploration of the suspected leakage site, involving the detection of preoperative intrathecally applied fluorescein, is more sensitive and can be helpful in precisely localizing the fistula, but this method may also entail some risks.4
Chemical analysis of the discharge fluid is a safe, noninvasive method. Protein and glucose are present in several body fluids in characteristic concentrations. In the past, these parameters have therefore been used as crude indicators for the presence of CSF. The protein β2-transferrin is found almost exclusively in CSF. Only a few body fluids, such as perilymph in the cochlea and the aqueous and vitreous humor of the eye, contain low concentrations of β2-transferrin.5 β2-Transferrin is the desialated form of transferrin, that is, it does not contain any molecules of neuraminic acid. This asialotransferrin can be separated from the other transferrin isoforms containing 1 to 7 neuraminic acids and can be demonstrated as a single band after isoelectrofocusing with transferrin-specific immunostaining.6 Detection of β2-transferrin thus allows indirect demonstration of extracranial CSF drainage if it is found in fluids known not to contain any β2-transferrin. Although it cannot provide any detailed information about the localization of the leak other than the right or left nostril, the presence of β2-transferrin in the analyzed specimen is strong evidence for the extracranial flow of CSF. Despite these facts, introduction of the β2-transferrin assay as a standard diagnostic tool has been slow, and other less specific or more invasive methods are used more widely as methods for screening for CSF leakage. The aims of the present study were therefore to investigate the clinical relevance of the detection of β2-transferrin in comparison with other diagnostic measures in a large series of patients with suspected otorrhea or rhinorrhea and, based on the results, to propose a scheme for the diagnosis of CSF leakage.
A total of 205 β2-transferrin assays were performed in 182 patients with suspected otorrhea or rhinorrhea at the Department of Otolaryngology, Medical University of Hannover, Hannover, Germany, between March 1995 and October 2001. The medical records of these patients were reviewed retrospectively to extract data, including age, sex, clinical findings, diagnostic measures, etiology of the clinical symptoms, treatment methods, and clinical course.
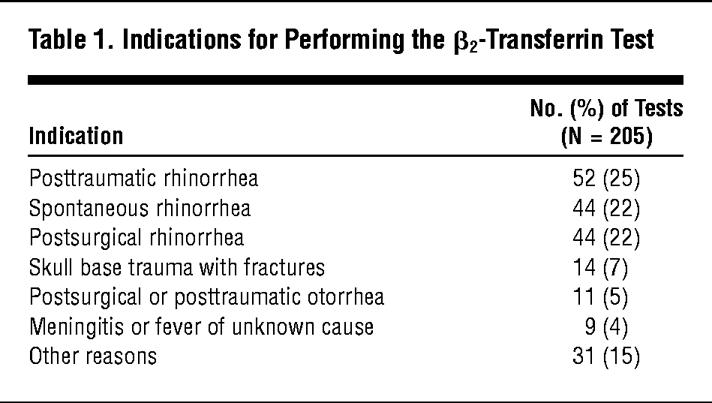
The main indications for performing the β2-transferrin assay fit into one of the following categories: unexplained spontaneous rhinorrhea or otorrhea, postsurgical rhinorrhea or otorrhea, posttraumatic rhinorrhea or otorrhea, trauma of the skull base, complex and individually selected cases of meningitis or fever of unknown origin, and other reasons (Table 1). Patient outcome has been defined based on patient clinical course. Patient outcome and surgical findings were used as the gold standard for positive or negative cases and have been summarized in Table 2.
Fluid suspected to be CSF was obtained as follows: direct collection or placement of Raucocel sponges (Lohmann/Rauscher International, Rengensdorf, Germany) in the nasal cavity or in the external ear canal to collect discharge fluid for 2 to 4 hours; intraoperatively with sponges or using a syringe; or out of dressing material or a surgical drain. For collection of the fluid from dressings or sponges, the material was placed in a funnel, which was inserted in a tube and centrifuged for 20 minutes at 6000g (Figure 1). A serum sample was always sent to the laboratory to be analyzed in parallel to test the specimen for β2-transferrin. If possible, fluid was sent immediately to the laboratory for analysis; otherwise, it was stored at 4°C maximally overnight.
Serum and secretory fluids were centrifuged at 6000g for 20 minutes. Total protein was determined by using dye binding assays such as Coomassie Blue or Pyrogallol Red (BioRad, Hercules, Calif). Glucose was determined semiquantitatively by means of test strips (MultiStix; Bayer Vital GmbH, Fernwald, Germany). Glucose concentrations greater than 0.3 g/L and protein concentrations up to 2 g/L were considered indicative of CSF.1 The concentrations of transferrin, prealbumin (transthyretin), and albumin were determined by kinetic nephelometry (Immage; Beckman Coulter, Fullerton, Calif). A prealbumin index was calculated by dividing the prealbumin ratio by the albumin ratio: PAB Index = PAB test:PAB serum/ALB test:ALB serum.
Xem thêm : ¿Cuál es la mejor melatonina para dormir?
Values greater than 1.5 were considered to indicate the presence of CSF.
For the β2-transferrin assay, serum and secretory fluids were diluted to equal total transferrin concentrations of 2 mg/L after saturation with ferric iron. Isoelectric focusing on agarose or polyacrylamide gels was used to separate the transferrin isoforms. Either immunoblotting (Figure 1) or immunofixation with antitransferrin and silver staining were used to isolate the asialotransferrin or tau band from other proteins and transferrin isoforms.6 Normalization of the total transferrin concentration to 2 mg/L provides the necessary uniform conditions for the immunostaining procedure and allows direct visual comparison of the intensity of the asialotransferrin band in the test fluids against the accompanying sera and a positive control (CSF) as illustrated in Figure 2.
Diagnostic imaging such as CT and magnetic resonance (MR) imaging showing any of the following indirect signs for a CSF fistula was considered positive: fracture lines, bony dehiscences, intracranial air following trauma, persistence or increase of intracranial air following skull base surgery with surgical opening of the meninges, protruding encephaloceles, or tumor formation at the skull base. Cisternography was used to demonstrate extracranial contrast or radiopaque medium after intrathecal application (Figure 3). Extracranial demonstration of intrathecally applied fluorescein in a subsequent surgical exploration of the skull base was considered to constitute direct evidence of leakage.
Standard descriptive methods were used to summarize the data. Frequencies and percentages were used for nominal variables, with means used for continuous variables. Sensitivity, specificity, and positive and negative predictive values were calculated for the β2-transferrin assay, CT and MR imaging, and glucose concentration.
During the study period (March 1995 to October 2001), 205 β2-transferrin assays were performed in 182 patients (mean [range] age, 44 [0-89] years) visiting the Department of Otolaryngology, Medical University of Hannover. Within the series, 110 male and 72 female patients were tested. In 20 patients, the β2-transferrin assay was repeated (17 patients received 1 repetition and 3 patients required 2 repetitions). Persistence or recurrence of rhinorrhea was the main indication to repeat the test (10 patients). Other reasons were to monitor success after a therapeutical intervention (8 patients) and recurrent meningitis (1 patient). In 1 patient, the test was repeated to confirm that bacterial contamination of the sample, and not the presence of CSF, gave rise to a positive asialotransferrin band.
The main indication for testing for β2-transferrin was posttraumatic rhinorrhea (n = 52 [25%]), followed by spontaneous rhinorrhea without obvious cause (n = 44 [22%]) and rhinorrhea following surgery of the skull base (n = 44 [22%]). Less frequent indications were skull base trauma with fractures of the temporal bone or anterior cranial fossa (n = 14 [7%)]), otorrhea after trauma or surgery to the temporal bone (n = 11 [5%]), and meningitis or fever of unclear origin (n = 9 [4%]). Other clinical findings (n = 31 [15%]) leading to the indication to test for the presence of β2-transferrin included conductive hearing loss due to effusion of unknown origin, wound drainage, or clear watery fluid from drain tubes after approaches extending to the skull base (Table 1).
Of the 205 β2-transferrin assays performed (examples are given in Figure 2), 35 results were positive, 169 were negative, and 1 could not be assessed owing to errors in the collection and handling of the samples (Table 2). Among the positive samples, CSF leakage was confirmed by surgery, cisternography, and the clinical history in 34 of the 35 patients. In 1 case, the specimen gave a positive result for β2-transferrin, but the examined fluid was judged not to be CSF. The fluid originated from a patient with chronic sinusitis and exhibited a suspicious green-yellow color. Because of the unusual appearance and the diminution of the otherwise dominant tetrasialotransferrin fraction, the examiner suspected contamination with neuraminidase-secreting bacteria, causing artificial desialation of transferrin isoforms. In view of the favorable clinical course and the other negative diagnostic findings, retesting was performed, confirming the absence of CSF in the nasal discharge of this patient. One test result was negative, although the patient’s clinical course corroborated the suggested CSF leak. A high-resolution CT scan showed a bony dehiscence in the lamina cribrosa, and the application of fluorescein and subsequent surgery revealed the leakage site. All other 168 negative test results were in accordance with the patients clinical state, with no signs of meningitis or persistence of otorrhea or rhinorrhea (test sensitivity, 0.97; test specificity, 0.99; positive predictive value, 0.97; and negative predictive value, 0.99). Table 2 compares the results of the β2-transferrin assay with surgical findings and case outcome, and Table 3 compares β2-transferrin assay with the results of other diagnostic measures.
Computed tomography was performed in 138 cases. In 46 cases, either a small fracture line, bony dehiscence, or intracranial air after trauma were identified and were considered an indirect indication of a possible dural defect (Table 2). Persistent or increasing intracranial air following skull base surgery with opening of the meninges necessitated further diagnostic evaluation, especially when the clinical course suggested a postsurgical CSF leak. Of 138 CT scans performed, 92 were without pathological findings. Taking the β2-transferrin results into consideration, only 13 patients tested positive using both diagnostic tools (Table 3). Eleven patients with a CSF leak tested positive for β2-transferrin, while the CT scan revealed no indirect signs of a CSF leak (test sensitivity, 0.58; test specificity, 0.71; positive predictive value, 0.30; and negative predictive value, 0.89).
Magnetic resonance imaging was used in 17 patients primarily for reasons other than detecting a CSF fistula (eg, diagnosis of tumors and encephaloceles or recurrence/residues of tumors), but the results suggested a CSF leak in 1 patient (Table 2). Surgical exploration confirmed the leakage in this patient. Of 17 MR images obtained, 16 revealed normal findings, although β2-transferrin test results were positive in 5 of these patients and the leakage sites were demonstrated by exploratory surgery in 3 cases, with the other 2 patients not treated surgically (test sensitivity, 0.20; test specificity, 1.0; positive predictive value, 1.0; and negative predictive value, 0.75).
Cisternography was performed in 2 patients using radioactive agents introduced intrathecally. Recurrence of symptoms led to cisternography being repeated in one of the patients; in this case, cisternography revealed a CSF leak (Table 2) and surgical exploration demonstrated dural dehiscence in the suspected leakage site.
Biochemical testing for glucose was performed in 19 patients, with 14 positive and 5 negative results (test sensitivity, 1.0; test specificity, 0.45; positive predictive value, 0.57; and negative predictive value, 1.0). Of the 14 glucose-positive cases, only 8 also tested positive using the β2-transferrin assay (Table 3). Leakage ceased in these cases following appropriate treatment. However, in the remaining 6 cases with positive glucose testing results, all other diagnostic measures as well as the clinical course did not indicate a CSF fistula (Table 2).
Protein concentration was measured in all samples tested for β2-transferrin. A low (<2 g/L) protein concentration indicative of CSF was obtained in 67 samples. This was not entirely consistent with the results of the β2-transferrin assay, revealing 20 positive and 47 negative results in patients who tested positive on the basis of protein concentration. In 23 patients, an elevated prealbumin index (>1.5) indicated the presence of CSF, and 22 of these were confirmed by the β2-transferrin assay (χ2 test, P<.01). However, the prealbumin index in the remaining 13 of the 35 β2-transferrin positive cases was not increased and therefore not indicative of CSF.
Twenty-one patients underwent surgical exploration; 15 of these procedures followed intrathecal application of fluorescein (Table 2). In 13 cases, exploration was prompted by confirmation of an assumed CSF fistula using the β2-transferrin assay. The leakage site was intraoperatively demonstrated in all these patients (Figure 4). Surgical exploration revealed the presence of a CSF fistula in 1 patient, although this individual tested negative for β2-transferrin (Table 2). The indication for surgical exploration in this patient was, as mentioned herein, based on a CT scan revealing a bony dehiscence of the skull base and on intranasal detection of intrathecally applied fluorescein. The remaining 7 individuals were explored surgically for treatment of fractures not related to a CSF leak, to revision surgery prompted by suspected CSF leakage (despite a negative β2-transferrin result), or to recurrent chronic sinusitis. In these 7 patients, no leakage site was identified during exploratory surgery.
Xem thêm : Right shoulder pain ICD 10 code
Management approaches varied depending on the clinical course, the diagnostic findings, and the localization of the CSF leak. Conservative treatment involving head elevation and a pressure bandage was provided to 15 of the 35 patients with a CSF fistula (confirmed by the presence of β2-transferrin). In 7 patients the application of a lumbar drainage device was sufficient to stop the leak, whereas in 13 patients surgical closure was required.
The identification of CSF-specific proteins such as β2-transferrin in patients with suspected liquorrhea seems to be a promising alternative to more invasive diagnostic procedures for establishing the diagnosis of a CSF leak. The initial method was first described in 19797 and has also found clinical application in the diagnosis of CSF leaks.8-11 Several modifications and refinements have enhanced the sensitivity of methods to identify β2-transferrin, and results can be available within 3.5 to 8 hours.12,13 Even low concentrations of CSF can be identified.11 Dilution effects caused by tears or other fluids play a less crucial role for β2-transferrin than for the measurement of glucose or protein concentration. Thus, patients with minimal or intermittent rhinorrhea or otorrhea in particular are allowed to collect fluid at the time when otorrhea or rhinorrhea is present and to send the specimen to the laboratory for analysis. This procedure improves the chances of identifying patients, including those with intermittent CSF leaks. In large clinical centers, the test is now a routine laboratory procedure and is frequently used for diagnosing a suspected CSF leak. However, in other clinics the test is not yet in routine use.
Sources of error in the use of the β2-transferrin assay have been analyzed.8,14 Provided the examiner is aware of the potential errors, this assay is a valuable diagnostic tool with high levels of sensitivity and specificity.8,14 The specificity of the β2-transferrin assay is enhanced by using isoelectric focusing in a highly standardized fashion. Using this method of refined β2-transferrin assay, we were able to achieve 99% specificity and 97% sensitivity. In 1 case, however, persistent rhinorrhea following endonasal sinus surgery and a postsurgical CT scan indicated a bony dehiscence, although the β2-transferrin test result was negative. Being aware that biochemical analysis of an assumed CSF leak is only possible when discharge occurs at the time the specimen is collected, the clinical course of the patient and the CT scan prompted us to apply fluorescein intrathecally. This finally revealed the leakage site during a subsequent surgical procedure. Although the β2-transferrin assay is highly specific, results have to be interpreted by an experienced reviewer, as was shown by the example of 1 patient with chronic sinusitis in which bacterial contamination of the sample was the reason for decomposition of sialated transferrin to the desialated form. It is therefore crucial to also provide clinical information about each patient to the laboratory performing the β2-transferrin assay. The prior measurement of the total protein concentration provides further valuable information regarding the possible origin of the fluid and eventual interference with the applied method. Serum samples were always analyzed face to face with the concomitant secretory fluids to recognize systemic elevations of β2-transferrin (Figure 2) in patients with alcoholic or nonalcoholic liver cirrhosis.15,16
Other diagnostic measures such as CT or MR imaging fail to demonstrate the leakage but might lead to the localization of a suspected leak if bony fractures and dehiscences, protruding encephaloceles, or tumors with bony erosions of the skull base are present. Intracranial air detectable by CT or MR imaging is an indirect sign of posttraumatic or erosive dural dehiscence or of surgical opening of the meninges. Therefore, in cases in which patients tested positive for β2-transferrin, a high-resolution CT scan of the region with the suspected leakage site should always be performed to gain valuable information for planning the surgical approach. However, as shown by the results of the present study, in some cases even high-resolution CT scanning fails to demonstrate bony defects, mainly because they are not dislocated or are too small to be imaged by this diagnostic tool. Treatment of a CSF fistula was required in 10 patients (surgery, n = 6; conservative treatment, n = 4) without pathological findings on CT scanning. In these cases, the management relied entirely on a positive β2-transferrin assay result and on the patient’s clinical course. In the 6 surgically treated cases, the CSF fistula was confirmed, localized, and closed.
More invasive methods, such as intrathecal application of radiopaque agents or fluorescein, are also available. These, however, carry potential risks, mainly neurological complications such as hemiparesis and seizures if the concentration of fluorescein is too high.4 Thus, in our experience the fluorescein concentration should not exceed 5% (1 mL). By modifying the original technique, it is therefore possible to reduce potential complications essentially.
In 15 cases, the clinical course was highly suggestive of CSF leakage, although 6 of these tested negative using the β2-transferrin assay and 1 was equivocal. Prior to a planned surgical exploration, fluorescein was applied intrathecally to investigate a possible leakage site. Five negative results subsequent to fluorescein application correlated with the negative results of the β2-transferrin assay. With the use of fluorescein application and surgical application, CSF leakage was ruled out in the patient who could not be assessed as positive or negative for β2-transferrin. However, in 1 of the 9 fluorescein-positive cases, the β2-transferrin assay failed to indicate CSF, although fluorescein application and surgical exploration revealed the leakage site. In this case, intermittent leakage and not the lack of sensitivity of the test was the most probable reason for the negative β2-transferrin result. The use of intrathecal application of fluorescein should be favored over the use of isotope or CT cisternography. Although all 3 methods are invasive in nature and associated with the mentioned risks, the latter 2 are less sensitive to detect a CSF leak. The advantage of intrathecally applied fluorescein is that the leak can be directly visualized, either by demonstration of the typically colored rhinorrhea or otorrhea or by endoscopic demonstration. In subsequent exploratory surgery of the skull base, it is possible not only to directly visualize the leak but also to seal it.
Biochemical analysis of suspicious fluids for glucose or protein concentration and CSF-specific proteins are noninvasive and harmless for the patient. However, studies have been published on the difficulties (caused by equivocal information based on confounding factors) associated with using protein or glucose concentration as indicators of CSF. These include, for example, the glucose content of tears or of mucous in normal or pathological nasal discharges.17,18 The large number of false-positive results explains why this tool was almost abandoned in the routine diagnosis of CSF leaks. However, protein concentration was determined routinely because it can be helpful in the analysis of the β2-transferrin content and the values obtained might provide an initial indication of the presence of CSF in a specimen. More recently, a rapid method for the identification of CSF has been introduced in the form of the beta-trace assay.19-21 Beta-trace protein had been identified as prostaglandin-D synthase.22 The main advantage of beta-trace is the extremely short processing time.23 However, several disadvantages have to be considered. In contrast to β2-transferrin, beta-trace is not a specific CSF protein. Only its high concentration in CSF makes it useful in the diagnosis of CSF leakages. Since contamination and dilution might occur—just as in the determination of glucose and protein concentration—misleading results might be obtained.
The data analyzed in this study were extracted retrospectively by performing a medical chart review. Considering the possibility of intermittent CSF leakage, patients with negative β2-transferrin results were advised to refer themselves to our clinic if symptoms did not resolve or if further symptoms appeared. In the group of patients with positive β2-transferrin test results, there was indeed 1 case of late meningitis that was referred back to us for further treatment. This case was initially surgically treated to close the CSF fistula and still had a persistent or recurrent CSF leakage. However, no patient dismissed with an initially negative β2-transferrin test result and with a clinical course not remaining suggestive for a CSF leak was referred back or was reported to us with late meningitis.
As with any other biochemical test, it must always be considered that the β2-transferrin assay can only prove the presence of CSF in the tested specimen, suggestive of a CSF fistula, but cannot directly demonstrate CSF leakage or the leakage site. To localize the CSF fistula, high-resolution CT scanning and intrathecal fluorescence application (with or without surgical exploration) may be essential. Differential diagnosis should be performed in cases in which patients tested negative for β2-transferrin and the possibility of allergic, vasomotoric, drug-induced, gustatory, endocrinologic, idiopathic, and multiple other forms of rhinitis and sinusitis—and indeed the role of psychopathological factors—should be carefully evaluated. If the test result is negative but the clinical course remains suggestive of a CSF leak, serial testing should be performed to exclude the possibility of sampling errors attributable to intermittent leakage. Careful imaging of the skull base to rule out any bony dehiscences or fine fracture lines is essential in such cases. Furthermore, the application of more invasive tests, such as intrathecal application of fluorescein, is justified if the history and clinical course remain suggestive of a leak despite all other diagnostic test results being negative.
All aspects considered, the β2-transferrin assay is an excellent method for the detection of CSF. Its high sensitivity and specificity is unequaled by other noninvasive methods. As an outcome of the present study, in our department we now use the β2-transferrin test as a primary screening procedure in all cases of suspected CSF leakage. Thus, unnecessary additional diagnostic procedures such as MR imaging and cisternography as well as glucose testing have been abandoned. However, CT scanning and facultative fluorescein application prior to a planned surgical procedure to localize the fistula continue to be essential elements of the diagnostic scheme.
Correspondence: Timo Stöver, MD, Carl-Neuberg-Str 1, 30625 Hannover, Germany (Stoever.Timo@mh-hannover.de).
Submitted for publication December 17, 2003; final revision received April 8, 2004; accepted June 10, 2004.
The authors thank Professor Dr Hartmut Becker, Head of the Department of Neuroradiology, Medical University of Hannover, for providing the radiological pictures.
Nguồn: https://blogtinhoc.edu.vn
Danh mục: Info
This post was last modified on Tháng mười một 26, 2024 4:25 chiều